Lecture Notes 34
Lecture 34 - Optimization
As part of the review on optimization, homework problem 19.14 was covered in class.
Homework Problem 19.14 from Process Dynamics and Control (Seborg, Edgar, Mellichamp, Doyle)
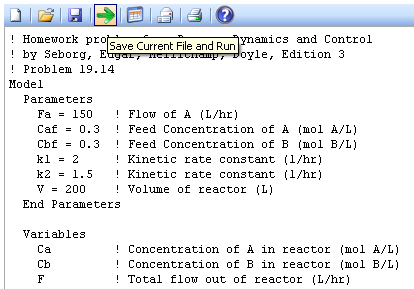
A reversible chemical reaction, A->B and B->A, occurs in an isothermal continuous stirred-tank reactor. The rate expressions for the forward and reverse reactions are:
- r1 = k1 CA
- r2 = k2 CB
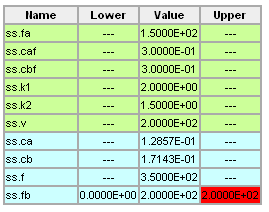
Using the information given below, use a numerical search procedure to determine the value of Fb (L/hr) that maximizes the production rate of Cb (i.e. the amount of Cb that leaves the reactor, mol B/hr). The allowable values of Fb are 0 <= Fb <= 200 L/hr.
Available information
- The reactor is perfectly mixed.
- The volume of liquid, V, is maintained constant using an overflow line (not shown in the diagram).
- The following parameters are kept constant at the indicated numerical values:
- V = 200 L
- FA = 150 L/hr
- CAF = 0.3 mol A/L
- CBF = 0.3 mol B/L
The solution to this problem is possible with a number of numerical or analytical techniques. One solution approach that we covered in class was with the APMonitor software through the web interface.
Equations and Solution to HW19.14
Equations for HW19.14
Solution to HW19.14